The Disc Replacement Book – By Jim Rider – The Evolution of Disc Replacement
So having an understanding of the complex and varying nature of the human spinal disc, and the entire spine, let’s take a look at how innovation and technology has evolved and worked through the process of solving this dilemma
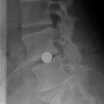
Fernström Ball, the first artificial “disc”
SB Charite
SB Charité III, was first developed at the Berlin Charite Clinic and produced in Germany by Waldemar Link Gmbh in 1987
Karin Büttner-Janz and Kurt Schellnack, under the supervision of Hartmut Zippel, developed a device now referred to as the SB Charite. This device had a sliding core design. The first two generations of the device worked well, but real success was had in the 3rd generation. The SB Charite disc was the first disc to be implanted over 1000 times. This device receive FDA approval for use in the U.S. in 2004 for single-level surgeries in L4-L5 or L5-S1. By then, the SB Charite was considered obsolete by many surgeons in Europe. Over a 20-year period, more than 30,000 SB Charite devices were implanted worldwide.
Prestige Cervical Disc Replacement 
Cervical devices were more difficult to develop than lumbar devices because of the smaller size required. The first successful cervical disc replacement to be widely used was the Prestige. Although it was developed and used as far back as 2003 and 2004, it did not receive approval by the U.S. Food and Drug Administration until 2007.
Other disc replacement implants were developed, including the ProDisc (lumbar and cervical), Maverick (lumbar), Active-L (lumbar), and Mobi-C (cervical). As of the writing of this book, the ProDisc and Activ-L have received U.S. FDA approval, but only for single-level use. The Mobi-C, and more recently the Prestige LP, have been approved for two-level use under limited conditions. The Charite Disc is no longer supported by its manufacturer. The Maverick was apparently barred from the U.S. market (at least initially) over patent infringement issues. Other devices continued to be developed.
This device is a “ball and socket” device, much like an artificial hip is a ball and socket. One piece containing a metal endplate and plastic ball is attached to one vertebra, and the other piece containing a metal concave end plate socket is attached to the other vertebra. The two halves are not physically attached to each other but rely instead on the pressure of the spine and surrounding tissues to keep the two parts in contact. The ProDisc has been used for more than ten years. This device was not approved by the FDA for use in the U.S. until 2006, and then only for single-level use.
Currently, in the U.S., the most commonly used device is the Pro Disc, which is approved for single-level use only. For a time, the Pro Disc was one of the best artificial discs available. But it has not aged well, so to speak. In fact, by the time the Pro Disc was approved for use in the U.S. by the Food and Drug Administration (FDA), it was already considered obsolete in Germany and other countries where disc replacement is frequently performed. Ongoing studies of the Pro Disc show a higher than necessary complication rate along with concerns for facet arthrosis. Unlike the M6 device, which mimics the normal, limited motion of a natural disc, the Pro Disc allows more than normal motion. This is one of those times when more is not better. The resulting hypermobility increases the stresses on the facets (portions of the vertebrae above and below the artificial disc). Over time, this can cause damage.
The Pro Disc has also had a rough track record when it comes to the need for revision surgery. In fact, studies have shown that the device has required as much as 8.7% of the patients to have a second surgery within two to three years (Siepe, Mayer, Wiechert, & Korge, 2006). Yet the ProDisc is still used for single-level disc replacement in the U.S. and Canada.
In 2005, Dr. Ritter-Lang and our team started working with the M6 device from U.S. based Spinal Kinetics. We had the honor of being the first to perform the very first implant of the cervical version of the M6 device in Europe. The Spinal Kinetics M6 was the first disc to truly mimic the motion of a natural disc, providing both motion and shock absorption. We also had the honor of assisting The Spinal Kinetics in developing the surgical tools and procedures for the The Spinal Kinetics M6 Disc Replacement. Since that time, we have installed thousands of the devices and have completed the first clinical studies charting two-year results within a large population of patients. We have also had the honor of training surgeons from around the world in the implantation of the The Spinal Kinetics M6.
At this time, we have installed thousands of the M6 devices. In fact, as of this writing, we have installed over 2,400 lumbar implants and more than 2,600 cervical implants. We have had excellent results with extremely low complication rates across the board.
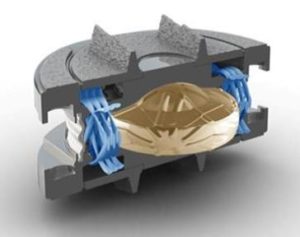
The Spinal Kinetics M6 Disc Replacement offers a “Quality of Motion” that is not present in any other implant we have seen!
Unlike early Disc Replacement designs, the Spinal Kinetics M6 artificial disc is designed to replicate the structure and performance of a natural disc. Its innovative design incorporates an artificial nucleus to allow shock absorption and a woven fiber annulus for graded variable motion resistance in all directions.
These characteristics accurately replicate the natural disc, allowing the implant to work in concert with the remaining human discs. Unlike earlier “ball-in-socket” implants, with the M6 disc replacement the resulting natural functionality of the entire spinal curve will provide the best chance for a full recovery. In addition, future complications will be eliminated by reducing adjacent level degeneration and strain on the muscles and ligaments.
Engineered to replicate your own disc, the M6 disc replacement is the only artificial disc that incorporates an artificial nucleus (made from polycarbonate urethane) and a woven fiber annulus (made from polyethylene). The M6 artificial disc nucleus and annulus are designed to provide the same motion characteristics of a natural disc.
Together, the M6’s artificial disc nucleus and annulus provide compressive capabilities along with a controlled range of natural motion in all 6 degrees of freedom along each vertebra.
This “natural” motion is designed to provide the freedom and stability you need to move naturally.
The M6 disc replacement has two titanium outer plates with keels for anchoring the disc into the bone of the vertebral body. These outer plates are coated with a titanium plasma spray that promotes bone growth into the metal plates, providing long term fixation and stability of the disc in the bone.
Next Chapter – Surgical Options – Fusion – The Gold Standard? > >
The Disc Replacement Book – A Back in Motion
By Jim Rider
About the Author
Motion – A Natural State
The Evolution of Disc Replacement
Surgical Options – Fusion – The Gold Standard?
Disc Replacement Procedure – Dr. Jan Spiller, Chief Orthopedic Surgeon
Disc Replacement Studies
Disc Replacement FAQ
Disc Replacement Surgeons
Disc Replacement Success Stories
Stenum and Back – My Disc Replacement Story – 2003
Free MRI Review