Advancements in Artificial Disc Replacement
 Background on Artificial Disc Replacement
Background on Artificial Disc Replacement
History of Artificial Disc Replacement, since the 1960’s, spinal surgeons have been artificially replacing damaged discs in the spines of patients. Since that time, medical researchers have been searching for the best product to replicate a spinal disc. Finding a product with the strength and flexibility to replicate the disc safely has proven difficult.
The discs that rest between the bones in your spine serve a unique purpose. They act as shock absorbers between the bones to allow both fluid movement and soften the jolts that occur in everyday life, from normal walking to sudden jumps.
Recent Artificial Disc Replacement Advancements in the US
Advancements in technology have given away to a new product, introduced on the US market in 2005. Called the ProDisc Artificial Disc for the cervical (neck) and lumbar (low back), it uses a three-part system to act and react just like a vertebral disc. There is also the LDR Mobi C that has been FDA approved for two levels of cervical (neck) disc replacement.
Characteristics of the these older Artificial Disc Replacement products include:
- An upper plate that attaches to the vertebra above the disc
- A hard plastic middle insert made of ultra-high molecular weight polyethylene that mimics the moving cushion interior of a healthy disc
- A lower plate that attaches to the vertebra below the disc
The plates are composed of cobalt chromium molybdenum and are sprayed with special titanium plasma that encourages bone growth. The idea is that once you heal, the surrounding bones will grow to envelope the plates, sealing in the artificial disc replacement. In “theory” you’ll be good as new once you recover from the procedure. Not so Much…
ProDisc Artificial Disc Replacement Approval
In 2009, the Food and Drug Administration (FDA) approved the ProDisc Artificial Disc. The ProDisc-L, for lumbar disc replacements, and the ProDisc-C, for cervical disc replacements, were approved for use in the United States. However, the procedure is still struggling to gain wide success and many surgeons are not yet offering the new product.
ProDisc underwent 11 years of testing prior to being released on the market. One study showed that 95 percent of the patients given a ProDisc artificial disc showed no problems even after 11 years had passed. The pain was gone, range of motion had returned, and there were no relapses. As reported by the Department of Neurosurgery at the University of California at San Francisco, the study reported that the ProDisc Artificial Disc was a success.
Artificial Disc Replacement News
Spinal Kinetics announces completion of 50,000 M6 Artificial Spinal Disc Implants
Recently Spinal Kinetics reached completion of its 50,000th M6 Artificial Disc Replacement implant.
Dr. Karsten Ritter-Lang has placed over 6,000 ADR implants and was the first to implant the Spinal Kinetics M6 disc replacement.
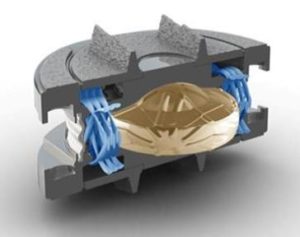
Unlike early Disc Replacement designs, the Spinal Kinetics M6 cervical artificial disc is designed to replicate the structure and performance of a natural disc. Its innovative design incorporates an artificial nucleus to allow shock absorption and a woven fiber annulus for graded variable motion resistance in all directions.
These characteristics accurately replicate the natural disc, allowing the implant to work in concert with the remaining human discs. Unlike earlier “ball-in-socket” implants, with the M6 disc replacement the resulting natural functionality of the entire spinal curve will provide the best chance for a full recovery. In addition, future complications will be eliminated by reducing adjacent level degeneration and strain on the muscles and ligaments.
Spinal Kinetics M6 disc replacement offers a “Quality of Motion” that is not present in any other surgical solution we have seen!
Unlike early Spinal Fusion Spinal Kinetics M6 disc replacement is designed to replicate the structure and performance of a natural disc.
Spinal Kinetics M6 disc replacement characteristics accurately replicate the natural disc, allowing the implant to work in concert with the remaining human discs. With Spinal Kinetics M6 disc replacement the resulting natural functionality of the entire spinal curve will provide the best chance for a full recovery. In addition, future complications will be eliminated by reducing adjacent level degeneration and strain on the muscles and ligaments.
This “Quality of Motion” is a major patient benefit with M6 Artificial Disc Replacement!
M6 Artificial Disc Replacement offers an innovative option compared to other surgical solutions like fusion surgery or discectomy because of its unique design, which is based on the qualities of the natural disc.
The M6 Artificial Disc Replacement is the only artificial disc that incorporates an artificial nucleus (made from polycarbonate urethane) and a woven fiber annulus (made from polyethylene). The M6 Artificial Disc Replacement nucleus and annulus are designed to provide the same physiologic motion characteristics of a natural disc. Extensive biomechanical testing with the M6 Artificial Disc Replacement lumbar disc has demonstrated equivalent Quality of Motion compared to the healthy disc.
Together, the M6 Artificial Disc Replacement’s artificial nucleus and annulus provide compressive capabilities and a controlled range of natural motion in all 6 degrees of freedom. This “natural” motion is designed to provide the freedom to move your back naturally.
The M6 Artificial Disc Replacement has two titanium outer plates for anchoring the disc into the bone of the vertebral body. These outer plates are coated with a titanium plasma spray that promotes bone growth into the metal plates
